|
Scientific Debate
Current status of lung protective ventilation in ARDSRobert A. Lachmann, MSc, Jack J. Haitsma, MD, and Burkhard Lachmann, MD, PhD. Department of Anaesthesiology, Erasmus Medical Centre Rotterdam, The Netherlands. |
||
|
||
|
Introduction ARDS was mentioned in an historic article by David Ashbaugh and colleagues in 1967. They described 12 patients with severe dyspnoea, tachypnoea, cyanosis, loss of lung compliance and diffuse alveolar infiltration seen on the chest X-ray [1]. They observed and reported several clinical and pathological similarities with neonates with respiratory distress syndrome, notably surfactant dysfunction [1]. Over 40 years before the work of Asbaugh’s group, Kurt von Neergaard in 1929 was the first to suggest that surface tension plays a role in lung elasticity [2]. He showed that the pressure necessary to fill the lung with liquid was less than half the pressure needed to fill the lung with air. His explanation for this remarkable difference was based on the assumption that in each alveolus there must be a barrier between air and fluid. This barrier could reduce the size of the alveolus according to the law of Laplace [2]. From the law of Laplace, P = 2y/r (P = pressure in the bubble; y = surface tension; r = radius of the bubble), it could be concluded that a reduction of the radius of a bubble needs an equal reduction in surface tension to keep the bubble stable, which can only be accomplished by a dynamic behaviour of a surface tension lowering material, which is pulmonary surfactant. Thus, when the endogenous surfactant system is impaired, independent of the cause, the rise in surface tension will result in atelectasis formation, enlargement of the functional right-to-left shunt, pulmonary oedema, impaired gas exchange and subsequent hypoxemia. These patients require mechanical ventilation to decrease their work of breathing and reverse the life-threatening hypoxemia and their respiratory acidosis. What did we learn from the last ten years of mechanical ventilation? In 1990 Hickling and colleagues demonstrated that mechanical ventilation could influence mortality in ARDS patients [3]. Lowering tidal volume (TV) in a retrospective study of 50 ARDS patients decreased mortality [3]. The outcome of this study sparked renewed interest in lowering TV in ARDS patients. Three subsequent controlled trials using low TV strategies were simultaneously started but all failed to improve patient outcome [4-6]. These studies used a TV of approximately 7 ml/kg in their low tidal volume arms and a TV of 10 ml/kg in their control arms [4-6]. In contrast, using a TV of 6 ml/kg in their treatment arm and a TV of 12 ml/kg in their control arm (TV calculated by using predicted body weight) the U.S.- ARDS network-study was able to reduce mortality [7]. The explanation given by the ARDS network trial for the beneficial effect on mortality was the greater difference in tidal volume between the two arms of the study, the power of the study (ARDS network studied 861 patients while the other 3 studied a maximum of 120 patients), and the aggressive treatment/ prevention of acidosis [7]. The only other randomized controlled trial to show a reduction of mortality in ARDS patients had been published 2 years earlier. Amato et al. reported that mortality in 53 patients was significantly reduced by applying a protective ventilation strategy [8]. In their study TV was also reduced to below 6 ml/kg in the low tidal volume group compared to 12 ml/kg TV in the control arm. In contrast to the three negative studies [4-6] the PEEP level in the low tidal volume group of Amato et al. [8] was significantly higher i.e. almost 17 cmH2O compared with 8-10 cmH2O PEEP in the studies by Brochard et al. [4], Brower et al. [5] and Stewart et al. [6] (Fig 1). Experimental data have shown that ventilation with low tidal volumes by itself does not prevent lung injury and may even worsen lung injury when repeated collapse of lung tissue is not prevented. In the ARDS network trial the low TV group had a slightly higher set PEEP of 9 cmH2O compared to a set PEEP of 8 cmH2O in the control group [7]. However, the increased respiratory rate (to help prevent acidosis) used in the low TV group may have resulted in intrinsic PEEP which contributed to a higher total PEEP (16 cmH2O) in this group [9] compared to 12 cmH2O in the traditional TV group. This higher total PEEP could help explain the decrease in mortality observed in this group. 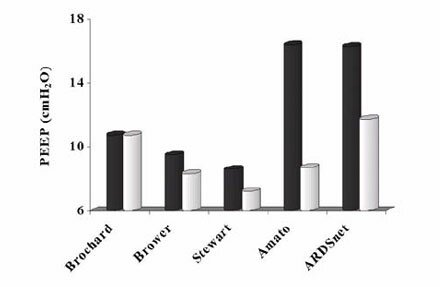 Figure 1 Total PEEP levels applied in recent studies on protective mechanical ventilation. Studies used are by Brochard et al. [4], Brower et al. [5], Stewart et al. [6], Amato et al. [8] and the ARDSnet [7] with intrinsic PEEP modification from De Durante et al. [9]. Black bars represent the PEEP levels in the lung protective strategies and the white bars the PEEP levels of the control arms of the corresponding studies. PEEP levels currently employed in intensive care units around the world are below 6 cmH2O in 78% of the patients receiving mechanical ventilation [10]. Even more disturbing is that in the same study only three patients of the 1638 ventilated patients studied had a PEEP level above 15 cmH2O [10]. Whereas it is known that PEEP levels above 15 cmH2O are necessary to prevent repetitive collapse of alveoli and thus reduce shear forces [11]. Furthermore, only studies using PEEP levels above 15 cmH2O in their protective arm have demonstrated a reduction in mortality [7-9]. WHY DO PATIENTS WITH ARDS DIE? Although ARDS is characterized by PaO2/FiO2 ratio below 200 mmHg in the American-European Consensus conference on ARDS [12], patients do not die from hypoxemia but rather die from multi-organ failure [13]. Ranieri and coworkers in 2000 linked increased levels of serum inflammatory mediators to organ failure in patients suffering from ARDS [14]. These increased serum levels of inflammatory mediators were observed in patients ventilated with conventional ventilation, in contrast a lung protective ventilation strategy (high PEEP, low TV) that minimized the inflammatory response and subsequently had a lower incidence of organ failure [14, 15]. Ventilation can induce mediator release especially in susceptible lungs (e.g. inflamed). Increased levels of cytokines in the serum were also observed in the ARDS network trial, in which higher levels of interleukin (IL)-6, a pro-inflammatory cytokine, were observed after 3 days of ventilation in the control arm compared to the reduced tidal volume [7]. Similarly, the number of days without non-pulmonary organ or system failure (circulatory, coagulation and renal failure) was significantly higher in the group treated with lower tidal volumes [7]. Increased levels of inflammatory mediators correlate with the development of ARDS and high broncho- alveolar lavage levels of these mediators in ARDS lungs have been described extensively. Furthermore, persistent high levels of inflammatory mediators in the lung over time correlate with poor outcome [16]. Similarly, plasma levels of inflammatory mediators correlate with severity of ARDS and subsequently outcome [16]. Headley and co-workers investigated the role of inflammatory plasma cytokines during infections and systemic inflammation and the subsequent development and progression of ARDS [17]. The final outcome of ARDS patients correlated with the magnitude and duration of the host inflammatory response in the serum and was independent of the precipitating cause of ARDS or the occurrence of infections [17]. Similar observation were made in multiple trauma patients in which high concentrations of cytokines correlated with the development of ARDS and finally multi-organ failure. Our group has demonstrated that injurious mechanical ventilation can result in loss of a compartmentalized inflammation response and thus increasing serum levels of inflammatory mediators [18]. Especially in the early stage of an inflammation the response will be compartmentalized, as observed in community-acquired pneumonia [19]. Because of the strong correlation between circulating inflammatory cytokines (especially TNF-a) and poor outcome during systemic inflammation, it has been suggested to decrease circulating levels of this cytokine (TNF-a) either by monoclonal antibodies or soluble receptor antagonists [20]. However, in all clinical trials in which these treatments were used mortality did not decrease and sometimes even increased. Although none of these trials were specifically aimed at ARDS patients, they included patients with ARDS. Thus, the intra venous infusion of some specific antibodies alone will not be the optimal solution to decrease the mortality of our ventilated patients. In healthy patients no effects on plasma levels of mediators were observed during 1 hour of mechanical ventilation; even ventilation with high tidal volumes on ZEEP did not result in higher cytokine levels compared with lung-protective ventilatory strategies. Previous lung damage seems to be mandatory to cause an increase in plasma cytokines after 1 hour of high tidal volume ventilation. Thus, in ARDS there is an inflamed lung with increased levels of pro-inflammatory mediators, and ventilation itself can increase the amount of inflammatory mediators produced by the lung. When the barrier function of the alveolar-capillary membrane is lost this will result in leakage of mediators to the circulation (decompartmentalization). The subsequent increased levels of these mediators in the circulation correlate with multi-organ failure and finally mortality. Use of lung protective ventilation in both experimental and clinical studies has demonstrated that a reduction in cyclic collapse of the lung reduces the amount of inflammatory mediators in the systemic circulation which in turn reduces organ failure and mortality. Lessons for the future In conclusion, in an ARDS lung or a lung that is susceptible to develop ARDS a higher level of inflammation is present. When these lungs are mechanically ventilated, ventilation that will increase the inflammation response should be minimized and the barrier function of the lung should be preserved. Especially ventilation with large tidal volumes combined with end-expiratory alveolar collapse and the subsequent appearance of shear forces should be minimized. With these guidelines, circulating levels of inflammatory mediators can be reduced which can help reduce the incidence of multi-organ failure in ARDS patients and finally reduce mortality. To minimize the effects of ventilation-induced lung injury the preferred ventilation should be pressure-controlled ventilation. When ventilating in a pressure-controlled mode the risk of overdistension of healthy parts of injured lung areas (as present in inhomogenous lung injury such as ARDS) is prevented. One should use as small as possible tidal volumes in order to prevent overdistension and dangerous shear forces and the latter should be combined with sufficiently high levels of PEEP to prevent endexpiratory collapse. Sufficient levels of PEEP will also help to prevent further loss of surfactant in still ‘healthy’ alveoli, halting the further spread of the disease process and reducing capillary leakage and transfer of cytokines, bacteria and other inflammatory stimuli across the alveolar capillary membrane. Finally, active recruitment of collapsed lung tissue should always be considered not only to improve oxygenation but also to reduce shear forces between the coexistence of collapsed and non-collapsed alveoli [21-23]. In 1992 Lachmann suggested such a ventilation strategy [23]. Using these guidelines and applying them to the lessons learned in the last 10 years we can further improve the outcome of ARDS patients and reduce the effects of iatrogenic lung damage. REFERENCES
Prof. Dr. Dr. B. Lachmann Department of Anesthesiology (Room EE 2393), Erasmus Medical Center Rotterdam, PO Box 1738, 3000 DR Rotterdam, The Netherlands. Phone:+31-10-4087312, Fax:+31-10-4089450 E-mail: |
Latest News
Deadline for submitting abstracts for EACTA 2012 extended until 22nd January 2012» Click here
18th National Congress of the Cardiovascular and Thoracic Anesthesia and Intensive Care Society
12-15th April 2012
Bodrum Turkey
» Read more
EACTA NEWS LETTER
October 2011
» Click here
EACTA on Linkedin
» Click here
EACTA ICU on Facebook
» Click here
15th Annual Comprehensive Review & Update on Perioperative Echo
February 6 - 11, 2012
San Diego, CA
» Read more
17th Annual Update on Cardiopulmonary Bypass
March 11 - 16, 2012
Aspen/Snowmass Village, CO
» Read more
Thoracic Anesthesia Symposium
April 27 - 28, 2012
Boston, MA
» Read more
34th Annual Meeting & Workshops
April 28 - May 2, 2012
Boston, MA
» Read more
Cardiothoracic Symposium
Iguazu Falls, Argentina
March 19th-21st 2012
» Read more
Abstracts from EACTA 2011
Abstracts from EACTA 2011 are published online by the Journal of Cardiothoracic and Vascular Anesthesia. Please go to:
» www.JCVAonline.com
2012 Combined Meeting of the 13th International Congress of Cardiothoracic and Vascular Anaesthesia and the New Zealand Anaesthesia Annual Scientific Meeting, to be held 14 - 17 November 2012, Auckland, New Zealand.
» Read more
EACTA Perioperative Echocardiography DVDs now available
Buy your copy as an EACTA member for €50.
» Read more
JCVA

The Journal of Cardiothoracic and Vascular Anesthesia (JCVA) is now the official journal of the European Association of Cardiothoracic Anesthesiologists (EACTA)......
» Read more
ACTA Autumn Meeting
Holywell Park
Loughborough, Leicestershire
16th - 18th November 2011
» Homepage
» Brochure
